- Joined
- Dec 17, 2007
- Messages
- 3,390
- Reaction score
- 4,403
Hello!
I have a question concerning the delineation of a retroperitoneal sarcoma.
This patient has a dedifferentiated RPS, the biopsy was performed in the solid mass in the upper left abdomen. It's a high-grade liposarcoma, not metastatic.
However, as you can see, there is a considerable mass, which likely represents well differentiated sarcoma, reaching very far into the pelvis.
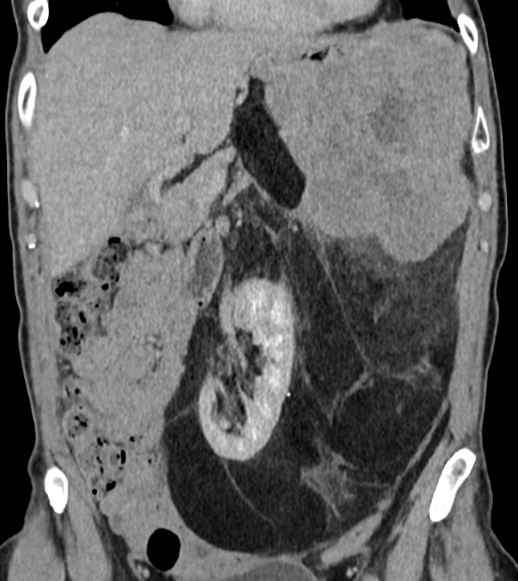
Neoadjuvant treatment is planned with chemo + RT, followed by surgery.
Would any of you consider
- to irradiate only the solid tumor component in the upper abdomen and ignore the rest
- to deliver a lower dose to the presumed well-differentiated component than in the solid component. This would resemble a bit the HR-CTV-approach, where higher doses are pursued at the projected resection margins and lower doses are accepted in non-critical areas, in order to reduce doses to OARs.
Published guidelines do not really distinguish this, and I understand that the risk of dedifferentiation is everywhere and not 100% corresponding to what we see on imaging. On the other hand, delivering 45-50 Gy to the entire extent of the tumor will be quite toxic.
Thoughts?
I have a question concerning the delineation of a retroperitoneal sarcoma.
This patient has a dedifferentiated RPS, the biopsy was performed in the solid mass in the upper left abdomen. It's a high-grade liposarcoma, not metastatic.
However, as you can see, there is a considerable mass, which likely represents well differentiated sarcoma, reaching very far into the pelvis.
Neoadjuvant treatment is planned with chemo + RT, followed by surgery.
Would any of you consider
- to irradiate only the solid tumor component in the upper abdomen and ignore the rest
- to deliver a lower dose to the presumed well-differentiated component than in the solid component. This would resemble a bit the HR-CTV-approach, where higher doses are pursued at the projected resection margins and lower doses are accepted in non-critical areas, in order to reduce doses to OARs.
Published guidelines do not really distinguish this, and I understand that the risk of dedifferentiation is everywhere and not 100% corresponding to what we see on imaging. On the other hand, delivering 45-50 Gy to the entire extent of the tumor will be quite toxic.
Thoughts?