- Joined
- Apr 21, 2011
- Messages
- 3,627
- Reaction score
- 9,284
Any favorite dose/fractionation recipes for early stage kidney SBRT? Just starting to get some cases and wondering what others are doing out there.
My experience with HCC is when the referring sends you a patient expecting SBRT, they’re confused if the plan becomes 15-20 fractions, and somewhat skeptical.Random musing:
Over the range of α/β = 5 to 10, 50 Gy/5 fx equals anywhere from 67.5 to 75 Gy in 15 fractions. Depending on practice setting, and technology being used for treatment, a 15 fraction regimen could well reimburse more than the SBRT regimen.
Just sayin'.
My experience with HCC is when the referring sends you a patient expecting SBRT, they’re confused if the plan becomes 15-20 fractions, and somewhat skeptical.
I usually do 40-45/5. At one point CSH was doing 36/3 and said they were getting good results but I never saw them published so…Any favorite dose/fractionation recipes for early stage kidney SBRT? Just starting to get some cases and wondering what others are doing out there.
That's what I've seen- stability of the tumor on imaging. Fortunately haven't had any recur yet.How are you surveilling them post xrt? If it’s anything like perc ablation, they don’t regress all that much and first signs of failure come from de novo contrast enhancement.
What factors are you looking at in planning an RCC/who is not a good candidate for it? Any concerns for messing up renal vasculature for central tumors? Any effect of mass size on treatment efficacy? Concerns for stricture or fistula if near the renal pelvis? Any major constraints from surrounding structures (i.e. anterior tumors adjacent to colon, pancreas, superior adjacent to spleen, etc? I know the data is likely too limited for any real controlled analysis, but looking for the overall gestalt. What is the impact of SBRT on renal function of the surrounding tissue? I.e. if you have a centrail tumor will you also be knocking off a good amount of functional parenchyma? Also the classic teaching is RCC = radioresistant, where is the dogma from?
This I can help you with. The short answer is what most people mean when they talk about how sensitive or resistant to radiation a tumor is they mean the how much (or little) radiation it takes to kill a given tumor type. Prostate is a great example. Following "standard" fraction sizes of around 2 Gy per day we deliver around 80 Gy for prostate cancer. That is a very high dose compared to things like anal SCC (50-54 Gy) or NSCLC (60 Gy) that we treat with primary radiation.RCC = radioresistant, where is the dogma from?
I have treated several solitary kidneys and never put someone on dialysis yet (most recent was about 6 months ago). Typical rise in creatinine is around 0.2-0.5 after 6-9 monthsI was able to keep a pt off dialysis who already had a prior right nephrectomy by treating her exophytic RCC of the L kidney with SBRT. Her SCr increased a bit to around 1.9, but stabilized there.
Also the classic teaching is RCC = radioresistant, where is the dogma from?
Dear DocTwoB:Prostate is a great example. Following "standard" fraction sizes of around 2 Gy per day we deliver around 80 Gy for prostate cancer.
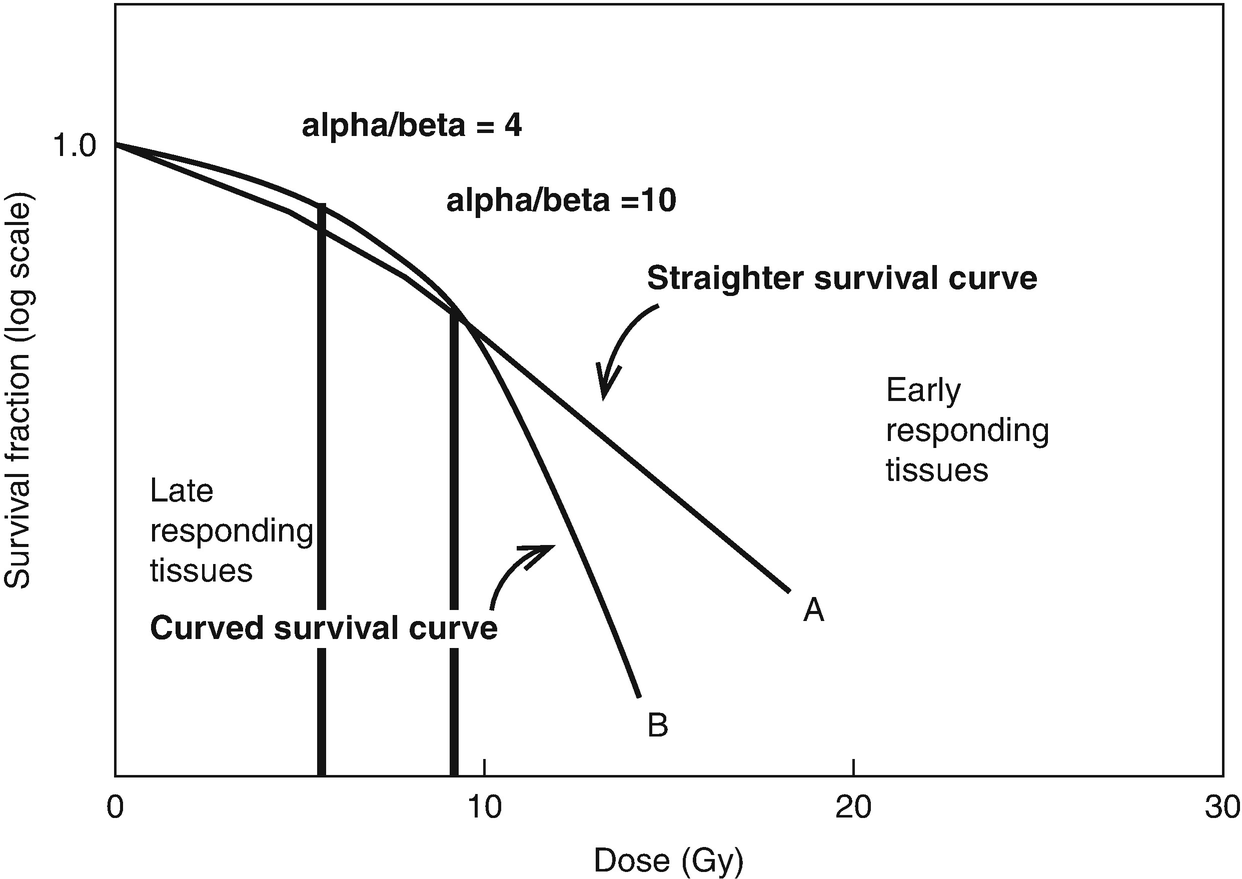
Dose escalation aside, 70 Gy is still >54-60 Gy. Yes, the prostate can and does move to some extent but that accounts for a small fraction of the difference between say an anal SCC and prostate cancer. Biology plays a role here and the dose response curves you show below below are much closer to reality. Grow yourself up some PC3 cells along with a few colorectal or NSCLC lines of your choice and generate your own curves. You provided the more complex explanation of what is meant by context dependent with respect to radiosensitivity...and why it is generally outdated.Re: ramses, all of the "dose escalation is good" data in prostate cancer came from a pre-IGRT era. The prostate is known to move versus its initial position at simulation in a fashion which can make systematic (versus random, i.e. like in lung) targeting errors more likely. Systematic errors necessitate ~3 times larger "PTV fudging" versus random errors. All of the data suggesting "much higher doses of RT are needed in prostate," versus other cancers, probably reflected the fact that several of the 40-45 prostate RT fractions "air balled," whiffed, missed, etc etc, before daily online IGRT. Old timey prostate dose escalation = fraction escalation = more opportunities to hit the target.
