- Joined
- Sep 2, 2003
- Messages
- 9,013
- Reaction score
- 4,743
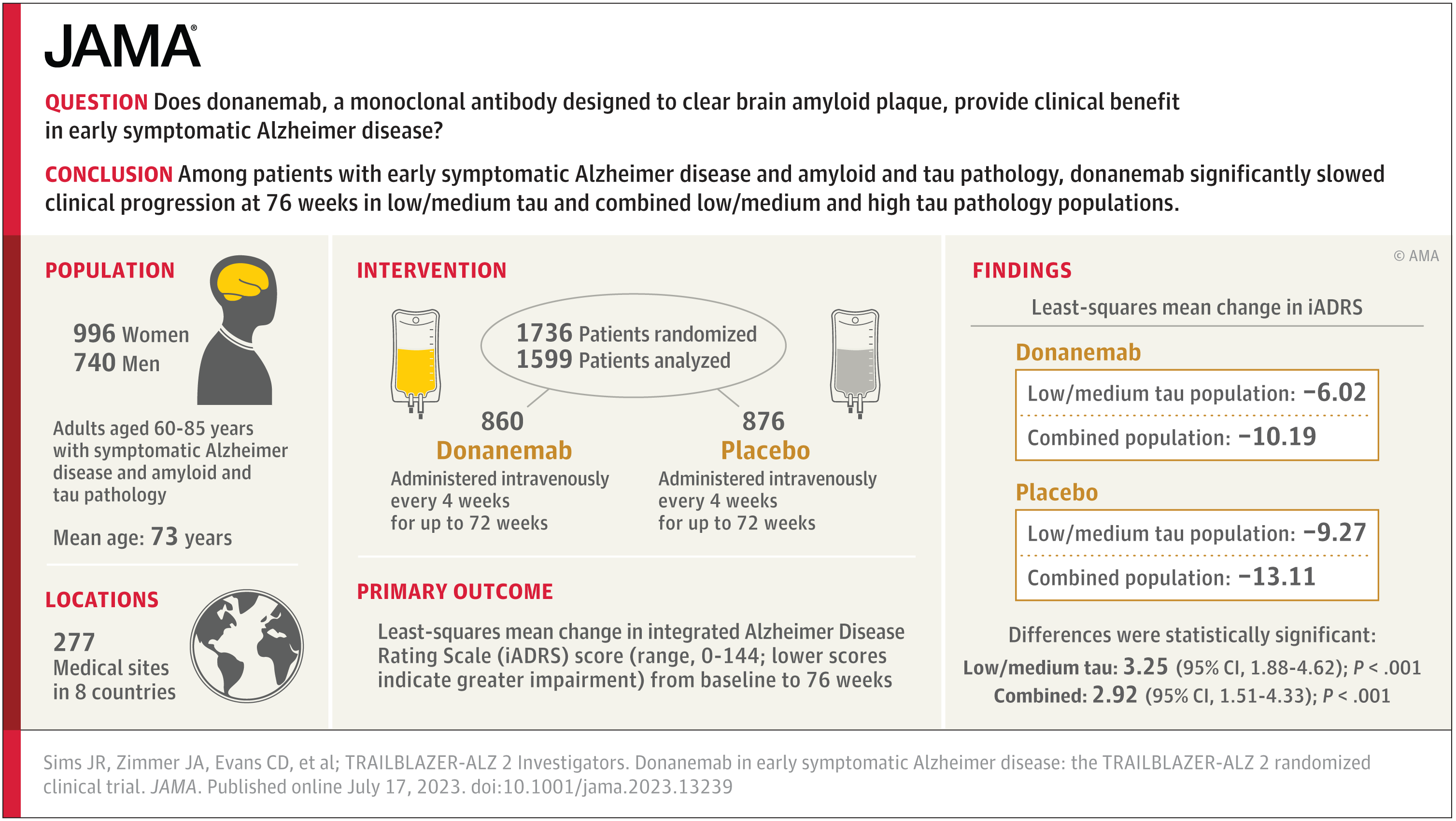
It didn't change my perspective all that much. My perspective on adu was that it probably did have an effect, but given a) the small magnitude of effect, b) the very significant adverse events in a non-trivial fraction of participants, and c) the messiness of the way the trials were conducted, we needed another study at minimum with unambiguous results before it would be worthy of approval. While lec isn't the same drug, it also had more robust patient selection criteria, and so I'm guessing that this is why the outcomes data is cleaner. Had Biogen run this study with adu instead and gotten the same result as they did with lec, I'd be fine with adu being approved right now. I still don't think lec is a particularly good option for use across the board given the low magnitude of benefit and still significant ARIA incidence, but it may be reasonable to use with careful patient selection.
"reasonable likelihood" should never be the basis of drug approval outside of something like a humanitarian device exemption. The last minute shifting of approval standards to seemingly whatever Biogen wanted them to be is exactly the problem here.
Maybe, maybe not - RCT with this as the endpoint or GTFO. If you haven't yet learned to replace blind enthusiasm with healthy skepticism regarding assumptions like this when it comes to the effects of AD trials, then you truly are a lost cause.
Although you open the door for aducanumab to have an effect, but you would prevent others from using it. You either total disregard for the autonomy of patients and peers. Weird control issues.
Reasonable likelihood is the threshold for accelerated approval. That's the FDA choice, not the advisory board. This happened with many meds, including Northera. Only 10% of accelerated approvals are later withdrawn, 70% go onto full approval.
Look, you've been totally wrong in this thread. You've been insulting to others who don't drink your kool aide by holding them in "contempt," you've called this a "travesty," and come up with unfounded charges of "data manipulation." Stop digging, start learning.