- Joined
- Jun 1, 2011
- Messages
- 628
- Reaction score
- 869
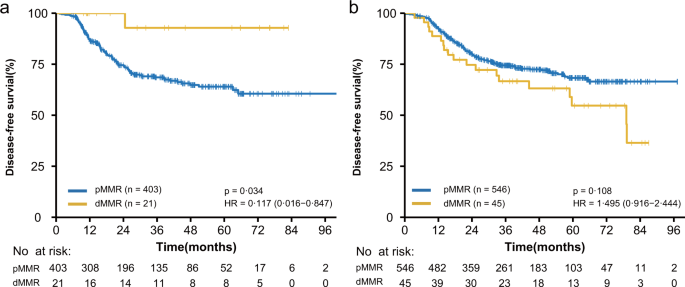
Has anyone seen the latest NCCN rectal guidelines showing neoadjuvant/definitive immunotherapy for MSI high patients as category 2B recommendation. Does anyone know what data this recommendation is based on? I'm hoping it's not that 12 person study.